

The report will also help all the prospective readers to identify major restraining factors for the industry participants.
STRYKER ICAD DRIVERS
Key drivers are discussed in the report, along with its impact on the growth of this industry during the historic period as well as throughout the forecast years. The report also provides an in-depth analysis of different drivers, restraints and opportunities in the ICAD (intracranial atherosclerotic disease) pathology Market. The global ICAD (intracranial atherosclerotic disease) pathology market was estimated at XX (USD Million) in 2020 and is projected to be valued at XX (USD Million) by 2026 at a CAGR of XX%.
Additionally, the report includes the study of opportunities available in the ICAD (intracranial atherosclerotic disease) pathology market on a global as well as regional level. The study includes drivers and restraints for the ICAD (intracranial atherosclerotic disease) pathology market along with the impact they have on the demand over the forecast period. The study provides historic data from 2016 to 2019 along with a forecast from 2020 to 2026 based on revenue (USD Million).

The report covers forecast and analysis for the ICAD (intracranial atherosclerotic disease) pathology market on a global and regional level. Mark-Paul, president of Stryker’s Neurovascular division said: “The unprecedented low complication rate shows that endovascular treatment may play an important role in optimizing clinical outcomes for patients suffering from this highly complex disease.To Understand How COVID-19 Impact is Covered in this ReportīUY NOW or REQUEST SAMPLE to avail Detailed Analysis Work still needs to be done to ensure complete routing of the disease. The results are commendable suggesting further development as compared to previous Trials confirming its approval and usage for treatment to assuage the disease. Investigators view the Trials as a crucial shift in the selection of patients for treatment by physicians. Using approved stents in the brain arteries may give new hope to patients suffering from a stroke due to blockages from cholesterol plaque.”
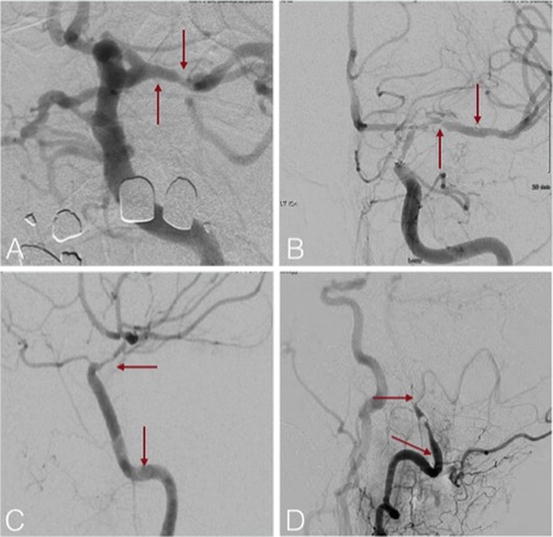
STRYKER ICAD TRIAL
Michael Alexander, director of the Neurovascular Center at Cedars-Sinai in Los Angeles and principal investigator of the trial, noted, “These trial results have the potential to change how stroke patients are treated in the future. Results signified patients receiving on-label treatment with the Wingspan Stent System demonstrated a 2.6% observed rate of stroke or death, compared to the pre-specified rate for early success, which was established as 4.0% with a minimum 150 patients. These results are significant when compared to the study’s null hypothesis with high predictive probability (>95%) that the true rate is 9.7%.ĭr. Stryker’s Wingspan StEnt System Post Market SurvEillance Study ( WEAVE Trial) as presented at the International Stroke Conference, testified the results for endovascular treatment with Wingspan Stent System for patients afflicted with intracranial atherosclerotic disease (ICAD) as the treatment set to alleviate the disease. WEAVE Trial is a multi-center, prospective, post-market surveillance study designed to assess the rate of stroke or death within 72 hours of the procedure in patients treated with the Wingspan Stent System.


 0 kommentar(er)
0 kommentar(er)
